For decades there has been a lot of debate over the idea that bodyweight is regulated. Decades of research suggests that there is a biological setpoint although others feel that a bodyweight settling point is a more accurate description of the system. Likely both play a role.
An additional question is whether it is bodyweight or body fat that is being regulated. Likely both play some sort of a role. However, inasmuch as body fat levels tend to be the most impacted by a diet, it makes sense that a primary signal to the brain of what was going on would reside in the body fat. The question then became what it was or even might be.
It would be decades later that the answer, or at least a partial answer would become apparent. That answer was leptin.
Table of Contents
The Eventual Discovery of Leptin
With early research (I’m talking the 1950’s) having established the existence of some type of setpoint (again, primarily in animal models), early researchers had to sort of guess what might be going on in terms of regulating body fat levels.
Essentially they postulated that the brain of the animal must be responding in some form or fashion to a hormone that scaled with body fat levels. They could only postulate what it was and it would take another 40 years before a major candidate would make itself known.
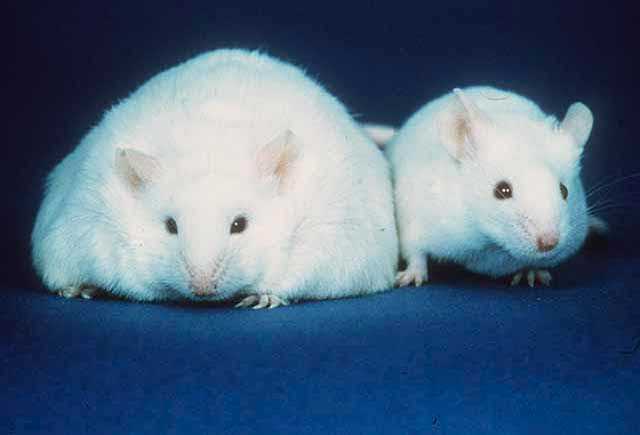
In 1994, the gene for a hormone that would eventually be called leptin (from the Greek “leptos” for thin) was discovered in the OB (OB stands for obesity) mouse. The OB mouse had been studied for decades and was spontaneously overweight with a low resting metabolic rate, low levels of activity, etc. It ate a lot, put on fat easily, etc. Here’s what it looks like compared to a normal lean mouse.
Superficially, the OB mouse appeared to be similar to obese humans (except furrier).
It turns out that the OB/OB mouse doesn’t produce leptin at all, it has a gene defect and makes zero leptin.
If you inject that mouse with synthetic leptin and it loses weight and fat rapidly.
After the discovery of leptin, the news was abuzz with thoughts that the cure for obesity was finally here. Companies spent a lot of money getting the rights to leptin, thinking it would fix the global obesity problem and they’d make zillions of dollars.
Leptin Levels in Humans
So researchers went about measuring blood levels of leptin in humans of varying weight expecting obese humans to produce no leptin.
To their dismay, it turned out that obese individuals invariably had very high levels of leptin and it was suggested that, in a similar vein to insulin resistance (where the body no longer responds appropriately to the hormone insulin), the body or brain had become leptin resistant. There was plenty of leptin floating around but it wasn’t sending the right signal to the brain to turn off appetite and reduce body fat.
I’d note in this regards that two other rat strains, the DB (for diabetic) and DIO (dietary induced obesity) rat show varying degrees of leptin resistance (the existence of resistance to the supposed regulating hormone was also postulated back in the 50’s). In the case of the DB rat, it’s complete and genetic; in the DIO rat it develops with increasing obesity.
A variety of things induce leptin resistance including high blood triglyceride levels and even leptin itself; when elevated chronically, leptin induces resistance to itself.
I’d note that it is currently being debated if leptin resistance is truly the cause for what’s going on and other models, such as the leptin insufficiency theory are being discussed as well; in this concept, a lack of leptin in the brain (but not in the body) is the problem. In either case, the signal from leptin isn’t being sent properly. I’ll talk about what that signal is in the next post.
And while a handful of individuals have been found who produce no leptin (and who respond to injectable leptin with massive weight loss and a normalization of metabolic rate), studies which injected leptin levels in the obese showed disappointing or no weight loss.
Which doesn’t make leptin useless, mind you; it was simply being used incorrectly because researchers didn’t quite understand what it was actually doing or supposed to be doing. Many people still don’t.
Insulin and Other Factors
Before wrapping this up, I want to note that leptin isn’t the only candidate hormone for body weight regulation; as it turns out insulin is also a key player here (insulin also scales with bodyfat). Direct injection of insulin into the brains of animals reliably reduces food intake and bodyweight.
There is also evidence, which I’ll discuss later, that there is a gender difference in how the brain responds to either leptin or insulin. Given that leptin scales mostly with subcutaneous fat (generally higher in women) and insulin scales mostly with visceral fat (generally higher in men), this will turn out to make some logical sense.
Of course, there are other factors here as well. Hormones such as cholecystokinin, peptide YY, ghrelin as well as blood glucose, blood fatty acids, amino acids, and others being discovered damn near daily are all sending an integrated signal to the brain about what’s going on in the body.
As well, varying hormones work on relatively longer or shorter time frames. For example, insulin can change in a matter of minutes, leptin may take hours, ghrelin operates on a meal to meal basis, etc. This makes for a very complicated system. But I’m getting ahead of myself.
What is Leptin?
Leptin is a protein hormone released primarily from fat cells although skeletal muscle, the gut and possibly the brain releases it too. But, in terms of overall quantity, fat cells are the primary place where leptin is synthesized and released.
Note: those of you still laboring under the false idea that fat cells are simply inert storage cells need to get out of the 1970’s and get up to date. Fat cells are turning out to be an endocrine organ in their own right, releasing a host of hormones and chemicals that have effects all over the body; leptin is but one of them.
Quite in fact, leptin scales scarily well with body fat percentage, as I noted on Wednesday, primarily with subcutaneous body fat percentage. The higher the level of body fat, the higher the leptin level and vice versa. Males below 10% body fat may have no detectable leptin in their bloodstream.
I’d note that, probably for hormonal reasons, women generally have 2-3 times as much leptin as men at any given level of bodyfat. There is also some evidence for gender differences in how leptin responds in women versus men to things like diet and exercise; more importantly, women’s brains may respond to leptin differently than men.
Tangentially, I suspect that this may be part of what’s involved in terms of why women generally have a harder time losing fat (a topic I discussed in some detail in my Bromocriptine booklet and that I’m delving even more heavily into right now).
Diet and Leptin Levels
However, leptin doesn’t only scale with body fat percentage, it is also related heavily to food intake, specifically carbohydrate metabolism in the fat cell.
In response to both over- and under-feeding, leptin changes quite rapidly.
When someone starts a diet, leptin may drop by 30-50% within about a week, obviously they haven’t lost that much of their body fat. After that rapid initial drop, drops in leptin are much slower scaling with body fat loss.
By the same token, with even short-term overfeeding, leptin can come up far more quickly than body fat is gained. This latter fact is part of the basic premise behind refeeding and cyclical dieting; short-term very high carbohydrate/caloric intakes can raise leptin without causing significant fat gain.
I’d note that, in the short-term, only carbohydrate intake affects leptin leptin levels. Fat overfeeding has no effect. In addition, changes in fat mass per se don’t regulate leptin in the short-term (less than 48 hours). Rather, it’s the effect of glucose metabolism within the fat cell that is affecting leptin synthesis and release.
This is why my diets always base refeeds around periods of high-carbohydrate intakes, acutely this is the only way to affect leptin levels in the short-term.
What Does Leptin “Tell” the Brain?
In essence, leptin is telling your body two different things:
1. How much fat you’re carrying.
2. How much you’re eating.
From the standpoint of bodyweight regulation and physiology, these are important things for the body to know about.
I want to note again that, as I mentioned in the last post, insulin is also a player in bodyweight regulation, scaling primarily with visceral fat and there is evidence that men’s and women’s brains are relatively more or less sensitive to the two hormones.
Women’s brains appear to respond more to changes in leptin while men’s respond more to insulin. As you’d expect, these effects are probably mediated by differences in hormone levels and it appears that estrogen improves the sensitivity of the brain to leptin. While not tested in humans, estrogen injected into male rats increases the response to leptin.
As I discussed in a previous research review, there is also evidence that estrogen exerts a leptin like signal in the brain as well.
I’d mention that, from a practical standpoint (regarding refeeds), this doesn’t particularly matter in that both leptin and insulin will primarily be increased via high-carbohydrate refeeds.
In any case, leptin (and insulin and, of course, the other hormones I mentioned last time) are sending a signal to the brain about body fat levels and food intake, making them likely candidates for bodyweight regulation. So how are they working exactly?
What Does Leptin “Do” in the Body?
Like most hormones in the body, leptin has effects nearly everywhere in the body. In skeletal muscle, it’s involved in promoting fat oxidation, it impacts on fat cell metabolism directly, liver metabolism, is involved in immune system function (which may be why dieters get sick when they get very lean) and more recent research is implicating effects on brain function, neurogenesis, breathing and a whole host of other stuff.
Of some interest, leptin levels are crucially involved in both puberty and fertility, it’s been known for decades that a certain level of body fat was required for puberty to hit and achieving critical levels of leptin appears to play a role in allowing puberty to begin.
The handful of folks who don’t produce leptin never hit puberty, for example and it’s thought that some of the reason children may be hitting puberty sooner is because increasing childhood obesity is causing them to hit that critical level sooner.
In a similar vein, leptin is a key factor in regulating fertility, essentially it ‘tells’ the body and brain that it’s well fed enough to spend calories on things like reproduction and making babies. This at least partly explains why dieters are very low levels of body fat lose both sex drive and the ability to function.
Loss of menstrual cycle is a well known effect of dieting and intensive training and while it was always thought to be related to body fat levels per se, it appears that energy availability (which, remember, leptin tells the body about) is a bigger factor. Essentially, when the body ‘senses’ that energy availability is insufficient, it shuts down what are essentially ‘extra activities’ such as reproduction.
In this vein, the most recent ideas about what leptin ‘does’ in the body are that it acts as an adipometer, a measurement of energy stores that tells the brain whether there are sufficient calories available to spend them on things like making bone, maintaining immune function, etc. Essentially the same concept I’m describing here.
My point being that leptin does a lot of stuff in the body, but that’s not mainly what I want to talk about here. Rather, in keeping with the theme of this blog series, I want to talk about leptin’s potential roles in bodyweight/bodyfat regulation.
Is Leptin an Anti-Obesity Hormone?
When it was originally discovered, leptin was originally conceived as an ‘anti-obesity’ hormone, it was thought that leptin should act to prevent weight gain. This led one researcher to quip (and I’m paraphrasing here) that “If leptin is meant to act as an anti-obesity hormone, it has to go down in history as the most ineffective hormone in the human body” or something roughly to that effect.
As I mentioned in previous blog posts, obese individuals invariably have high levels of leptin, raising levels in those folks does little to generate weight loss and because of that failure, everyone sort of moved on in terms of using leptin as a treatment for weight loss.
The problem is that early ideas about leptin were conceptually incorrect; rather than acting as an ‘anti-obesity’ hormone per se, leptin appears to act as more of an ‘anti-starvation’ hormone. That is, leptin doesn’t act to prevent weight gain, it acts to keep you from starving to death.
This reconceptualization would go a long way towards explaining the apparent assymmetry in the bodyweight regulation system I discussed previously: the body doesn’t defend against weight gain very well, it defends tenaciously against weight loss.
Various research found that the drop in leptin was a key aspect triggering (or at least mediating) the effects of starvation (dieting is just starvation on a smaller scale) in humans. In that vein, several studies had individuals diet before replacing leptin to pre-diet levels. This raised metabolic rate, normalized thyroid and increased fat loss.
Basically while trying to raise leptin in overweight individuals is pretty much a bust, preventing leptin from dropping on a diet (or raising it back to normal levels after weight has been lost) is where the real action is.
In this vein, recent work has found that females suffering from amenorrhea (a loss of menstrual cycle) respond to replacement levels of leptin with improvements in reproductive function, bone health, thyroid and overall hormonal axes, etc. Without weight gain.
So now you know basically what leptin ‘does’ in the body at least conceptually: it signals the brain about energy stores (both body fat levels and energy intake) and appears to act primarily as an anti-starvation hormone. Next time I’ll look at mechanistically some of what it does (e.g. impact on appetite, etc) and then about how to go about dealing with this on a diet.
Energy Availability and Leptin Levels
So when you are in an energy deficit and/or losing body fat, leptin levels drop.
Although I haven’t talked much about the role of exercise here I’d only note that whether or not the deficit comes from caloric restriction or exercise per se doesn’t appear to have much of an effect on how much leptin drops.
Basically, the body appears to be sensing “energy availability” (defined as calorie intake minus exercise energy expenditure divided by lean body mass) and adjusting things based on that. I’d, of course, note that exercise still plays plenty of other crucial roles (including psychological, which I am getting back to slowly but surely) in terms of dieting and fat loss.
What Happens Now?
Well, a bunch of stuff. Leptin interacts with various part of the brain but the hypothalamus (where the setpoint is primarily thought to be regulated) appears to be the key aspect. In conjunction with the other hormones I haven’t talked much about yet, when leptin drops a bunch of other neurochemicals change.
These all have complicated names like Neuropeptide Y (NPY), Agouti Related Peptide (AgRP), Pro-opiomelanocortin (POMC) and Cocaine Activated Receptor Transcript (CART). The names are not that important practically. When these hormones change, they cause other changes further downstream that affect all aspects of metabolism.
There are other regulators as well, in my little Bromocriptine booklet, I pointed out that brain dopamine levels go down when leptin goes down and this appears to play a role in the overall metabolic adaptation to dieting. The whole idea in that booklet was to use a dopamine agonist to ‘trick’ the brain into thinking it was fed, it worked for about half of the people who tried it; I’m still trying to determine what the cause of the variance was.
Lowered dopamine has a secondary effect that low leptin makes animals (mice and rats at least) more likely to addict to drugs when you starve them (there are other mechanisms at work here, of course): they need something to drive the dopamine/reward system. There is also evidence that obese individuals have impaired dopamine signalling in the brain.
In any case, POMC/AGRP/NPY/CART have further downstream effects and regulate things like metabolic rate (which drops when you diet), appetite/hunger (which go up when you diet), activity levels (you tend to get lethargic, burning less calories in daily activity), hormone levels (including thyroid via TRH/TSH and reproductive hormones via LH/FSH), etc. Testosterone and thyroid generally go down as does nervous system output, cortisol goes up. You get the idea.
Please note again that the extent of these changes depends to a great degree on the extent of the diet and the body fat level of the individual: someone dropping from 35% to 30% body fat might see only small changes (or almost none at all) in these parameters, someone who is getting leaner at the 15% range is seeing bigger problems and someone at 5% body fat (e.g. a natural male bodybuilder) is undergoing massive adaptation.
This is a big part of why dieting gets so much harder as people get leaner, muscle loss accelerates, hormones are crashing, etc. My Ultimate Diet 2.0 goes into much more detail on this topic.
Falling Leptin Triggers the Adaptations to Dieting
Basically, the body undergoes an overall adapatation that attempts to slow fat/weight loss (via reductions in metabolic rate and activity) and seek out food, these adaptations become stronger the leaner the individual gets (you’ll see that this has implications for how to fix it). I’d note that there is more to the overall adaptation to dieting than just the central effects in the brain; for example, impaired conversion of T4 to T3 in the liver is a well known effect of dieting.
Of course, various hormones have other peripheral effects in terms of energy balance and fat loss; for example leptin directly stimulates fat oxidation in skeletal muscle and a known adaptation to fat loss is a decrease in fat oxidation.
There is also that post-starvation hyperphagia I talked about in an earlier post, whereby signals from fat cells drive hunger to extreme levels when food is made available. Which, I’d note is pretty much always in modern society.
Note again (this ties in with my comments above) that the original observation of post-starvation hyperphagia was made in males who were kept on 50% maintenance calories for 6 months, ultimately reaching a body fat percentage of ~5% (that is, the lower limits of human body fat levels). Someone going from 35% to 30% isn’t going to experience nearly that effect and there’s going to be a continuum of responses from fatter to leaner that’s going to occur.
Finally (ok, probably not finally), leptin also impacts on how well or how poorly other appetite hormones in the body send their signals to the brain (that’s in addition to those other hormones sending a signal to the hypothalamus). For example, cholecystokinin (CCK) is a hormone released from the gut primarily in response to protein or fat intake; it’s involved in making you feel full after a meal. As is turns out, in rats at least, CCK doesn’t work as well when leptin is low.
Hardcore dieters (e.g. contest bodybuilders and figure/fitness competitors) are well aware of this: when they start getting very lean, even if they do everything ‘right’ at a given meal (i.e. lots of lean protein, moderate fat, fiber, moderate amounts of low GI carbs), they simply don’t stay full very long. Because all of the short-term fullness signals just aren’t working as well.
That’s because leptin is essentially setting the overall ‘tone’ of the brain in terms of how it responds to other signals. The various hormones that determine when you get hungry or full aren’t working as well when leptin is lowered from dieting and fat loss. Leptin certainly isn’t the only hormone involved in all of this; but it’s definitely one of the most important ones.
Finally, next time, what to do about all of this (short of not dieting and just staying fat and happy).
Summarizing What Has Gone Before
1. Human bodyweight appears to be biologically regulated, that is it makes some attempt (that can be overcome by environment, of course) to maintain body fat within some range or level.
2. The system regulating body fat is assymetrical, for most people it defends against fat loss much more strongly than against weight gain.
3. For proper regulation, the body needs a way of ‘knowing’ two things: how much fat you’re carrying and how much you’re eating; a variety of hormones play a role here.
4. At least in terms of indicating the amount of body fat is present, the hormones leptin and insulin appear to play a major role. Leptin scales with subcutaneous body fat levels (higher in women), insulin scales with visceral fat levels (usually higher in men); there is some indication of a gender difference in response to the different hormones.
Leptin and insulin also both change with changing food intake; leptin levels can drop significantly within a few days of dieting even with no change in body fat levels. Insulin changes meal to meal.
5. When people reduce calories and lose fat, leptin levels drop, and this appears to be a major part of the overall adaptations to dieting in terms of metabolic rate, hunger, etc.
While leptin certainly isn’t the only hormone involved it appears to be one of the major ones not only having direct effects but also impacting how well or how poorly other hormones (such as CCK) work in the brain.
6. While studies have found that raising leptin in overweight individuals typically does little (for reasons related to either leptin resistance or insufficiency in the brain), preventing leptin from dropping during a diet (or raising it) appears to reverse many of adaptations that occur.
Point 6 raises a question invariably comes up about now: why can’t I buy leptin?
Why Isn’t Leptin Commercially Available?
And the answer is that it has never (and I suspect will never) been made available outside of research. When I originally wrote my Bromocriptine booklet, an effective dose of leptin came in around $1000 PER DAY. The last time I looked (about a month ago), it’s down to about $500 per day. That’s assuming a chemical company would sell it to you.
That’s not a typo mind you, leptin makes growth hormone look cheap.
For various reasons, it simply hasn’t been developed for human use outside of research applications. Why? I can’t say for sure. I suspect it’s because drug companies primarily want weight loss drugs that cause weight loss and leptin doesn’t do that.
They don’t seem to want drugs that simply make dieting work better. I’d note that the average dieter isn’t looking for that type of compound either. Drugs that generate weight loss without the person having to change their behavior patterns are the real goal.
There is also the issue of leptin being a peptide hormone, meaning it would have to be injected. Injectable drugs are a bitch practically and there’s been a huge push to develop diabetic solutions not involving injectable insulin for that reason; the odds of the typical person injecting leptin twice daily while dieting are slim.
Bodybuilders would, of course, but that small percentage of people trying to get to 5% body fat are not the target market of the drug companies.
End result: nobody is developing leptin for commercial use so far as I can tell and I doubt this will change.
But for dieters and especially the very lean, injectable leptin would be a godsend fixing a majority of the problems that occur with dieting. Unfortunately, it’s a pipe dream at this point.
Now what?
Diet, Supplements, Training and Leptin
This leaves us with other approaches (e.g. nutritional, supplements, training) to attempt to manipulate either leptin levels or signaling.
There are basically three places where dieters might impact leptin levels and/or activity in terms of fighting off the adaptations to dieting.
1. Production at the fat cell
2. Signaling in the brain
3. Transport into the brain
Increasing Leptin Production
I talked a little bit about #1 in a previous post, when I talked about refeeds. At this point, and this topic is discussed to some degree in nearly every book I’ve written at this point, interjecting high carbohydrate, high calorie refeeds of varying lengths (anywhere from 5 hours to 3 days) is (currently) the best way to raise leptin while dieting.
One of the interesting (and often missed points) is that, as dieters get leaner (and leptin drops more and more), refeeds need to become larger and/or more frequent. That is, rather than necessarily dieting harder as they get leaner, some people are actually doing better by “breaking their diet” (with specific high-carb refeeds) more frequently.
I’d note again that leptin production is related primarily to carbohydrate intake in the short-term, high-fat refeeds aren’t the best way to raise leptin levels. I’d also note that single ‘cheat’ meals won’t impact on leptin levels significantly as leptin doesn’t really change on a meal to meal basis.
Tangent: I’d note that, in this regards, some of the work being done with intermittent fasting and every other day refeeds has relevance here as some data suggests that leptin may be maintained better with that approach to dieting.
An additional strategy, talked about in some detail in my Guide to Flexible Dieting is the idea of full diet breaks, periods of 10-14 days in-between periods of active dieting where calories are brought back to maintenance (and carb intakes brought back to at least moderate levels).
Not only does this provide a psychological break from the grind of continuous dieting, it helps to ‘reset’ some of the metabolic adaptations that occur with dieting. Leptin levels will come up, thyroid conversion in the liver is improved, etc. Assuming dieters have no strict time constraints, I strongly feel that inserting full diet breaks every so often (how often depends on body fat levels) is important for long-term success. Again, for both physiological and psychological reasons.
There are at least two other regulators of leptin levels here, both zinc and Vitamin E intake appears to regulate leptin production and I have suggested supplementation of both in the past to try to help raise leptin. How much (if any) impact this actually has I can’t say.
Increase Leptin Action in the Brain
Although it seems a bit out of order, I want to jump next to leptin activity in the brain. This is part of the area that gets generally referred to as ‘leptin sensitivity’ in the literature and is, unfortunately, poorly studied and even more poorly characterized.
What causes it, what (if anything) can be done about it is a huge question mark although finding ways to improve leptin sensitivity would probably also have huge benefits. Similar to improving insulin sensitivity, increasing leptin sensitivity would mean that the same level of hormone sends a larger signal. A supplement or drug that increased leptin sensitivity would be expected to do some very nice things.
I would mention that there is indirect evidence that regular exercise improves leptin sensitivity. I say indirect because measuring leptin sensitivity in humans is very difficult. Improved leptin sensitivity is being inferred from the fact that endurance athletes often have leptin levels below what you’d expect given their body fat level; this suggests increased sensitivity. Again, it’s hard to measure in humans.
It does appear that increasing levels of leptin induce resistance to itself (I’ll spare you the mechanism) so it’s conceivable that reducing leptin levels (e.g. with a diet) could transiently reduce leptin resistance/improve leptin sensitivity. How much of an effect or how long this would take is currently unknown.
If this were the case, would provide more support for cyclical dieting approaches such as my Ultimate Diet 2.0. During dieting periods, leptin levels would go down (but sensitivity would go up); during periods of deliberate overfeeding, improved leptin sensitivity (until such time as it went down again) could possibly be taken advantage of.
A similar logic could be applied to weight gain, eventually chronic overfeeding/weight gain might potentially induce leptin resistance; inserting periods of dieting to deliberately lower leptin might offset this.
While I’m on the topic, I should mention that leptin resistance can occur at other tissues such as skeletal muscle (I haven’t talked much about leptin’s actions there).In animals at least, both exercise and fish oils increase skeletal muscle leptin sensitivity.
Increase Leptin Transport Into the Brain
The final topic I want to talk about is that of leptin transport into the brain, something else I haven’t really talked about in this series. But it’s thought that leptin transport issues at the blood brain barrier may be part of the overall “leptin resistance syndrome” and impaired leptin transport into the brain may be part of the problem. It’s thought that leptin transport into the brain can become saturated, that is, once leptin gets above a certain level in the bloodstream, no more can be transported into the brain.
But leptin transport into the brain is also actively regulated by the blood brain barrier, by a variety of things, let’s look at a few:
High blood triglycerides tend to reduce leptin transport and it’s interesting to note that, despite being high in fat, low-carbohydrate diets often reduce blood TG levels. Is it possible that enhanced leptin transport part of the often observed appetite blunting effect that is often seen with these diets (along with other potential mechanisms of course)?
In a similar vein, high-carbohydrate diets, especially combined with low levels of activity often raise blood triglyceride levels, probably hindering leptin transport into the brain.
Both insulin and epinephrine increase leptin transport into the brain. Tying in with my comments above, this might be another reason that high-carbohydrate refeeds ‘work’ after a period of dieting; between (potentially) increased leptin sensitivity in the brain and insulin increasing leptin transport, there is a brief period where leptin signalling should be increased.
The supplements ephedrine and synephrine would be expected to increase leptin transport, ephedrine by raising epinephrine levels and synephrine by directly binding to beta-receptors.
And, of course exercise raises levels of epinephrine and, at least transiently should increase leptin transport into the brain. In that vein, quite a bit of research suggests that the body better regulates food intake when exercise is performed, increased leptin transport (and signalling) might be part of the mechanism.
And while I can’t find the paper now, I seem to recall a rat study suggesting that long-term (4 months if my memory isn’t failing me) fish oil supplementation could increase leptin transport into the brain. But it would likely take a very very long time to occur in humans.
And, at least for the time being that’s pretty much all I have to say about leptin. Next time, I’ll take a quick look at some of the other hormones involved in this system before (finally) moving onto some psychological issues that play a role in dieting.
Other Hormones At Play
While leptin absolutely plays a primary role in bodyweight regulation it is far from the only factor involved. I talked a little bit about insulin above but there are still more hormones of some importance. With more likely to be discovered as time goes on. Oxyntomodulin, GLP-1, PP and others are being discussed in recent reviews and further research will go towards determining what in the hell is actually going on.
Tangentially, this is one of the big problems in trying to find a true “solution” to the issue of weight loss and obesity: the human body has a number of overlapping, integrated and redundant pathways that all send signals to the brain. Fix one and something else eventually steps in to fill the role and cause problems.
From a pharmacological standpoint, this likely means that multiple drug therapy will be required; I also suspect that research and testing will help to identify whether any given individual has a specific pathway that is more of a contribution, perhaps allowing drug or nutritional therapy to be more individually tailored. However, this is likely to be years off from practical application.
Anyhow, I want to finish up by talking about a few of these other hormones focusing on three main ones: Cholecystokinin, ghrelin and Peptide YY as these three currently have the most research on them.
Cholesctyokinin
Cholecystokinin, or CCK, was one of the first fullness hormones found, originally discovered back in the late 1960’s. Released from the intestines in response to nutrient intake, it goes to the brain, binds to its specific receptor and helps to signal fullness on a meal to meal basis. CCK doesn’t appear to play much of a role in the long-term regulation of bodyweight, its simply a fullness signal in response to meals.
Nutritionally, protein, fat and fiber play a primary role in stimulating CCK with carbohydrate having a much smaller effect; this may explain part of the appetite blunting effect of many low-carbohydrate diets (which are generally high in protein, fat and fiber).
As I mentioned in a previous post, CCK doesn’t work very well when leptin is low explaining why lean dieters can do everything ‘right’ nutritionally and still be a hungry an hour later.
Ghrelin
Released primarily from the stomach, ghrelin goes to the brain where, predictable, there is a specific receptor. Among other functions, ghrelin raises levels of growth hormone. But that’s far from all.
Ghrelin also stimulates hunger (the only hormone so far found to do so) and appears to be a key hormone in initiating the hunger that goes along with meals; ghrelin drops prior to hunger and injection of ghrelin stimulates hunger specifically.
Even more interestingly, there is research suggesting that ghrelin levels become entrained to normal meal times.
So if you normally eat at 3pm (or whatever), you’ll likely find yourself becoming hungry at 3pm; this appears to occur from changes in ghrelin. I suspect this explains why people often have problems changing meal frequency, at least until ghrelin re-entrains itself to the new frequency.
That is, moving from a higher to lower frequency of meals is often accompanied by hunger at the previously ‘normal’ meal times. Moving from lower to higher is often accompanied by a lack of hunger until the body adjusts to the new frequency. I haven’t seen any work examining how long this takes but empirically it seems like it’s a couple of weeks or so.
Increased ghrelin also negatively impacts on pretty much all aspects of metabolism, slowing metabolism and increasing fat storage, at least it does this in rats with daily infusion.
In this vein, I’ve heard rumors that ghrelin is being promoted as a bulking aid for athletes and bodybuilders, both for the appetite increasing effects and the GH release. Given the negative aspects of ghrelin on metabolism, this is truly an awful idea unless the goal is to just get really fat.
In contrast, a ghrelin antagonist might be a very nice thing indeed for dieting. There appears to be work on orally available ghrelin antagonists going on.
As it turns out, ghrelin changes in the opposite direction of leptin; while leptin falls on a diet, ghrelin goes up. It almost goes without saying that leptin levels have a hand in controlling ghrelin. As well, leptin appears to restrain both grhelin release from the gut and its stimulation of hunger.
So dieting, as usual is a double whammy in this regards: leptin goes down as ghrelin is going up with the reduction in leptin being partly responsible for the increase in ghrelin.
Ghrelin appears to play a role in both short- and long-term hunger and long-term bodyweight regulation. As mentioned above, ghrelin goes up prior to a meal; it also comes back down after eating.
However, ghrelin levels also increase overall with a loss of weight/bodyfat, decreasing when weight is gained. Individuals with a high BMI have lower ghrelin (and the idea of ghrelin resistance has been thrown around) and anorexics have higher ghrelin (which decreases with refeeding).
Nutritionally, carbohydrates appear to play a primary role in regulating ghrelin levels with dietary fat having less of an impact, the effect of protein is currently unclear. In one study, a high carbohydrate/low-fat diet generated weight loss without the normal increase in ghrelin levels.
And although only tested in anorexics, at least one study showed that the consumption of non-caloric fiber reduced ghrelin levels. Consuming small amounts of guar gum or psyllium fiber between meals might help to keep ghrelin down during a diet.
Perhaps ironically, it appears that low-sodium intakes increase ghrelin levels (although there is a racial effect). Among other reasons, this is why the idea of reducing sodium excessively while dieting is a mistake.
In one study increases in ghrelin with weight loss were related primarily to fat free mass loss but not body fat loss per se. As good reason as any to ensure that the diet is set up to prevent lean body mass loss.
Of some interest, one of the ways that bariatric surgery appears to be so successful is that, despite the massive weight loss generated, there is often no increase in ghrelin levels as would be seen with diet induced weight loss.
This may explain why weight is so rapidly lost, seemingly without hunger, with that surgery. I’d note that research also suggests that other hormone (such as Peptide YY, discussed next, and Glucagon like peptide 1, are more relevant to the hunger suppressing effect of the surgery).
Peptide YY
Finally is peptide YY (PYY), another important hormone released primarily from the small intestine. Like CCK, PYY decreases hunger and appetite although it may do so for longer periods. Infusion of PYY blunts hunger in humans for up to 24 hours.
More physiologically, PYY increases with 15 minutes of eating and may stay elevated for up to 5 hours. Of some relevance to the issue of overweight, obese individuals have been found to have lower basal PYY levels and less of an increase with meals.
Of course, since this is all interconnected, administration of PYY has been shown to reduce fasting PYY levels as well as preventing the normal increase in ghrelin before meals.
Nutritionally, PYY appears to be related primarily to the energy content of the meal although work suggests that dietary fat has the biggest impact on PYY. The appetite supressing effect of protein appears to be related to increased PYY levels as well. Fiber increases PYY as well.
Of some interest, one study comparing a lowcarb/highfat to highcarb/low fat diet found that the lowcarb/high fat diet had a greater sustained effect on PYY levels in obese individuals.
All of the Others
The above only scratches the surface of all of the different factors that may send a signal of fullness or hunger to the brain. Blood glucose, amino acids, fatty acids, and many other hormones are all playing a role even if I’ve mainly focused on the primary ones.
Where Does This Leave Us?
As I mentioned above, there’s a lot of interacting and overalapping things going on when it comes to appetite/hunger and bodyweight/bodyfat regulation. Even looking at the above hormones it’s clearly complicated without worrying about leptin, insulin, blood glucose and everything else.
For example, one study finds that high carbs and low fat is better for supressing ghrelin while another finds that lowcarb and high fat has a bigger impact on peptide YY (which may be low in the obese to begin with).
Looking at individual macronutrients carbs have the largest impact in supressing ghrelin while protein, fat and fiber appear to have the biggest impact on CCK and peptide YY.
Is one hormone relatively more important than the other or is a moderate approach to dieting, where each meal contains all four macronutrients (plenty of lean protein, moderate fat, dietary fiber and moderate amounts of carbohydrates) going to be superior by targetting all of the gut hormones (in addition to providing the greatest dietary flexibility and variety)?
Is keeping ghrelin from going up relatively more important than increasing CCK and PYY or does it simply depend on the individual? If raising PYY with plenty of protein, fat and fiber not only helps with short-term fullness but also blunts ghrelin increases (as infusion studies suggest) does that avoid the whole issue since you accomplish both (increased PYY and lowered ghrelin) with the same intervention?
Does all of this lend more credence to the use of low-carbohydrate diets for the treatment of obesity? There’s an additional interaction of diet with insulin sensitivity as well where the optimal diet may depend on the degree of insulin resistance or sensitivity present.
As I noted above, my gut (ha ha, get it?) says that different individuals are going to be relatively more or less responsive to the different hormones.
If ghrelin is a bigger issue than the other hormones (and insulin sensitivity is good), then a high carb/low-fat diet may very well be the superior choice for a given individual. If PYY is dominant (and insulin sensitivity is poor), a low-carb/higher fat diet may be the best choice.
I’m not saying that I have the answers, I’m not sure anybody does at this point. As noted, I suspect that we will get to the point that basic testing of gut hormone levels, insulin sensitivity, etc. will get us to the point that diet optimization can occur. At that point things like diet, supplements, etc. can be tailored to the individual which will hopefully improve overall results.
Similar Posts:
- Insulin Resistance and Fat Loss
- Insulin Sensitivity and Fat Loss
- What is The Best Diet?
- Does the EC Stack Stop Working?
- A Guide to Calorie Partitioning
Comments are closed.